BASEL, Switzerland, April 13, /PR Newswire/ - At the 39th Annual Meeting of the European Association for the Study of the Liver in Berlin (April 14-18), Rochès commitment to finding solutions for hepatitis patients will be evident when 17 abstracts are presented, which demonstrate the efficacy and safety of PEGASYS(r) and COPEGUS(r) in hepatitis C and PEGASYS in hepatitis B.
Among the highlights are new findings from three ground-breaking global trials recently completed in patients with HIV-HCV co-infection,'normal’ ALT, and hepatitis B.
- APRICOT, the first and only global trial examining the role of pegylated interferon combination therapy (PEGASYS and COPEGUS) in patients co-infected with HIV and HCV will have its final results presented, including data on the predictability of treatment outcome from a patient's early virological response.
- For the many patients with HCV and persistently normal alanine aminotransferase (ALT) levels, new evidence will be presented about the positive impact of successful treatment on a patient's quality of life and what ability there was to predict treatment outcome early on in therapy; and
- The final results of a Phase III study of PEGASYS in HBeAg-negative hepatitis B will be announced, including the observation of HBV surface antigen loss - the most complete response possible to treatment and indicative of a complete remission. If left untreated, patients with HBeAg- negative HBV are more likely to develop severe liver disease than those with HBeAg-positive disease.
Interested journalists may view the text of abstracts selected for presentation, as well as the meeting schedule on-line at www.easl.ch.
per maggiori informazioni:
For more information, please contact: Sheila Gies, Roche, Tel: + 1 973 687 0188 (mobile), + 1 973 235 4347; Alison Thorpe, Burson-Marsteller, Tel: +44 20 7300 6324
Ultimi Articoli

Crans-Montana — Bertolaso: segni di miglioramento per alcuni feriti ricoverati al Niguarda

Al Maestro Silvano Cristofaro, un compleanno pieno d'arte.

Mahmood firma la canzone originale del nuovo film di Muccino “Le cose non dette”

Gli eventi principali di Triennale Milano dal 14 al 18 gennaio 2026

AtleticaMENTE, il doppio percorso che ispira: fra gli esami universitari e la maglia della nazionale

Olimpiadi, Milano introduce una procedura semplificata per le riprese foto e video in città

Hair, la tribe che parla ancora al presente

Crans-Montana — Palazzo Lombardia e Milano in lutto per le giovani vittime, Fontana ringrazia Meloni
Florida - Crime down 42%, more jobs, better schools – what Coral Gables did
Florida — il modello premiato dal Baldrige - 42% meno crimini, 20% meno disoccupazione – cosa ha fatto Coral Gables

Crans-Montana, rientrate a Milano le salme dei cinque italiani deceduti nella tragedia

Strage di Crans-Montana: la Lombardia in prima linea. Dal ponte aereo per i feriti al lutto per le vittime italiane

La guerra silenziosa della droga – Qualcosa si può fare!

Auto 2026: il motore che non gira. L'industria italiana è in folle ?

La Verità Sulla Droga — L’informazione scende in strada — Intensificata la campagna contro le bugie degli spacciatori

Milano, via Novate cambia volto — Housing sociale e un parco da 30mila metri quadri nel nuovo piano attuativo

Carte d'identità cartacee, Milano accelera il passaggio al digitale

Quello che vi hanno promesso sui tassi e il mutuo che costa ancora uguale
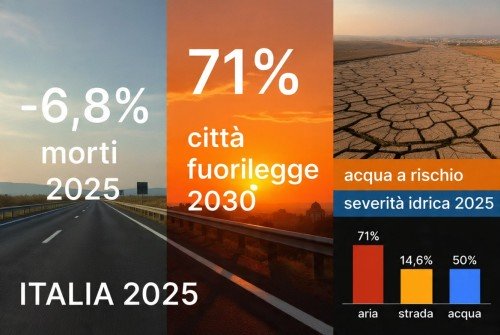
Il 2025 che abbiamo percepito contro i dati che raccontano davvero








